Our Work Portfolio:
‘Our Work’ demonstrates the depth and proficiency of N2VATE experience through several case studies. These storyboards illustrate an innovative and opportunistic apptitude and adeptness with the entrepreneurial activities of launching and branding products or companies in medical device, scientific instruments, and also retail health product spaces. We welcome the opportunity to answer any questions and discuss in greater detail how we may apply our combined experience in business development to your situation. Please contact us to discuss your needs and interests.
A success story for your product is waiting to be written with N2VATE, too. Let us define your success…
Retail Health, Product and Market Development: Diasan Corp


Exclusively licensed client’s IP, funded, and founded Diasan Corporation as CEO, serving the diabetes marketplace. Followed guidelines of the American Diabetes Association and created Elovate 15, a portable sachet of 15 grams of fast-dissolving fine powder glucose to help diabetics manage severe low blood sugar episodes. Created and branded Elovate 15. Developed market segments on Amazon, industrial B2B, and B2C direct online. Sold company March 2022 and advised for 12 months.
Elovate 15 Glucose Packs for Hypoglycemia Rescue
Don Kloos, Diasan Corporation, May 2019
Summary of “Elovate 15 Glucose Packs for Hypoglycemia Rescue” by Don Kloos, Diasan Corporation, May 2019
Elovate 15 Glucose Packs are designed for rapid and controlled relief from hypoglycemia. The human body relies solely on glucose for immediate energy, particularly critical for individuals with diabetes experiencing low blood sugar (hypoglycemia). Conventional sugary foods and drinks contain various sugars (fructose, sucrose) and complex carbohydrates that require digestion and conversion to glucose, causing delays and potential health risks.
Elovate 15 offers a direct and efficient solution by providing 15 grams of pure glucose in a portable, fast-dissolving powder form. This allows for quicker absorption through the mouth, bypassing the digestive process. Compared to sweets and drinks, Elovate 15 raises blood sugar levels about 60% faster, stabilizing within 15 minutes. The American Diabetes Association recommends the 15/15 rule for hypoglycemia: check blood sugar, consume 15 grams of glucose, wait 15 minutes, and recheck levels.
Elovate 15 is more convenient, cost-effective, and preferred over glucose tablets, gels, and liquids due to its quick dissolution, portability, and pleasant taste. It’s a practical and reliable option for immediate blood sugar correction in emergency situations.
Medical Device and Company Launch: NOMAD and Aribex Inc.


Aribex Inc.
N2VATE founder Don Kloos was tapped by long-time work friend, Dr. Clark Turner, to join him as a founding executive and minor investor to launch his medical device company, Aribex, and his invention: a hand-held, battery operated, dental x-ray source for intra-oral dental radiography. This was challenging from technical, regulatory, and marketing perspectives and required strong crusading to finally gain approvals and successful market traction. The following is an account of the process and Kloos’ activites and contributions:
Market Research and Validation:
Determined size and characteristics of the dental market, and surveyed dentists to understand objections and product appetite, among other parameters. Analyzed P&L that served as the basis for a pro forma for investors. Approached private investors and funding was successfully obtained.
A hand-held x-ray product created special regulatory, safety, and performance issues which required persistent collaboration with state regulatory agencies, universities, industry associations and sales channels to finally gain acceptance. Was it safe and did it work as well or better than wall-mount x-rays? The FDA, state agencies, sales force, and end-users were educated and convinced through intense crusading. The FDA 510(k) approval was obtained in approximately only two weeks after submission.
Product Promotion
Named the product “NOMAD” with a tag line of ‘Anytime…Anyplace’ to promote the benefits of mobility, convenience, and cost efficiency. Created and pitched a comprehensive market plan and budget to the board including: tradeshow and promotion, a new website, collaterals, including printed and electronic materiel, promotional and training videos.
Branding
Further branding and traction for NOMAD and Aribex was accomplished by engaging key opinion leaders to validate the safety and efficacy of NOMAD. In a strange twist of events, NOMAD gained international acclaim when it was sent to the Asian Tsunami zone which allowed international forensic dental teams to identify 4000 victims.
Results:
Aribex NOMAD has been successfully branded into the dental industry, worldwide, with sales revenues in the millions and thousands of users. A second generation product was subsequently developed and has been released for sale.
Testimonial
“Don Kloos was instrumental in helping establish Aribex as a start-up in the dental market. He did the market research to help us identify the market potential, and found the data regarding number of dentists and specialists that allowed us to prepare accurate pro forma estimates for our early investors. Don established our product branding, messaging, and oversaw the creation of our website and other marketing materials. He came up with our product name, NOMAD, which has been one of our most important trademarks! Significantly, he identified the major distributors and was able to get introductions and meetings with the key decision makers that allowed us to gain access to the dental markets, both domestically and internationally. He is a pleasure to work with, and injects a sense of humor with his strong work ethic, making a significant contribution to a start-up success.”
Dr. Clark Turner, President, CEO, Founder of Aribex Inc.
Market Development & Product Management: XRF Jewelry Karat Assay




Key Accomplishments and Attributes:
Displaying entrepreneurial vision to recognize a significant market opportunity in the jewelry trade, and then leveraging technology and marketing aptitude, acceptance of a new x-ray fluorescence instrumental analysis method was achieved for assaying gold karat fineness within the global jewelry industry. This initiated the opening of a new market niche and inspired $200M in worldwide commerce through 7000 XRF instrument installations to date. This required vision, planning, execution, and intense crusading and has inspired over 12 companies, including major scientific manufacturers, to engage in commerce worldwide in this niche market.
Market Discovery / Product Development and Launch:
Investigated jewelry industry market and found no active competitors. Researched market needs, provided technical specifications to Seiko Instruments. Launched the Seiko Instruments SEA-2001 Element Monitor, a benchtop energy dispersive x-ray fluorescence spectrometer, and the jewelry industry’s first practical solution to karat fineness assay.
Second Product Launch, CMI (now Oxford Instruments) 2000-2011(current):
Resourceful modification of existing XRF product line, to provide a less expensive, faster, and more accurate instrument. Launched CMI’s ‘Instant Assay’ into jewelry industry worldwide, in North America, Europe, Asia, and India. In all, worked with 5 XRF manufacturers and 6 products, including current (2011) design of an advanced instrument with Parallax Research.
Branding and Promotion:
Engaged in intense uphill branding, education, and promotion activities within the industry which included PR in the trade journals National Jeweler and American Jewelry Manufacturers Magazine, plus NBC Dateline (‘All That Glitters” 1995), San Francisco District Attorney’s Office / Consumer Fraud Division, and extensive personal work with many key opinion leaders (KOL) in the jewelry industry (see below). The new method was involved in several legal cases exposing and prosecuting underkarating jewelry manufacturers. See ‘Underkarat Jewelry, the Perfect Crime’. In the jewelry hub of 47nd Street in New York City, jewelers referred to the new assay method as ‘getting a Seiko’, much like photocopying documents is now referred to as ‘Xeroxing a copy’.
Key Opinion Leaders, Manufacturers:
Aribex NOMAD has been successfully branded into the dental industry, worldwide, with sales revenues in the millions and thousands of users. A second generation product was subsequently developed and has been released for sale.
- Michael-Anthony Jewelers, NY; Michael and Anthony Paolercio founders
- Stuller Settings LA; Matt Stuller, CEO
- OroAmerica, CA; Guy Benhamou, CEO
- Aurafin FL; Mike Gusky, CEO;
- Engelhard, CA; Joseph Ching
- SEEPZ, Bombay India; Multiple major jewelry manufacturers in this 'export zone'
Key Opinion Leaders, Trade Organizations:
- MJSA, Manufacturing Jewelers and Silversmiths of America, RI; Matt Runci and Jim Marquart CEOs
- Gemological Institute of America, CA; Dr. Jim Shigley
- London Goldsmith Hall, London England; Paul Johnson, Chief Assayer
- World Gold Council, NY; Michael Barlerin CEO
- Platinum Guild of America, CA; Jurgen Maerz
- Jewelers Vigilance Committee, NY; Cecilia Gardner CEO
- Lithuanian Assay Office, Lithuania; Eimantus Mitkus, Managing Director
Technical Articles & Presentations on X-Ray Fluorescence and Gold Jewelry Assay by Kloos:
Testimonial
“Don Kloos initiated the development and acceptance of the analytical technique, x-ray fluorescence, into the jewelry industry in the late 80’s. He assisted instrument manufacturers, jewelry manufacturers, distributors, retailers, and refiners in understanding the usage and benefits of the XRF method for this application. This made it possible for jewelry manufacturers to carefully control the gold karat content of fine jewelry that is needed to comply with quality regulations, and made the manufacturing process more efficient. This replaced and complimented the traditional fire assay technique which is destructive and much slower. He worked with the industry over several decades and the technique has now gained widespread acceptance and use worldwide.”
Anthony Paolercio, co-founder Michael-Anthony Jewelers, NY
A demonstrated vision for new opportunities, extensive entrepreneurial acumen, plus a contingent of strategic associates are available for you through N2VATE..…..How can we define YOUR success?!
New Product & Market Development: Veeco Micro X-Ray Analyzer




Background
While operating as a consultant for Veeco Instruments, a large read-write head customer in the data storage industry expressed a strong need for a thin film metrology solution that exceeded capabilities of all existing metrology tools. Recognizing the strategic opportunity to develop new business, applied knowledge and experience of x-ray and x-ray fluorescence technology to formulate a new micro x-ray fluorescence technique involving new x-ray focusing polycapillary optics, x-ray detector, and analytical software.
Key Accomplishments and Attributes
Proposed the new configuration and guided Veeco, their data storage manufacture customer, and component vendors towards this new solution. Provided and supported a temporary stop-gap solution for the customer while the new technology was being developed. The venture was successfully completed resulting in a new micro x-ray fluorescence technique and a high-end capital scientific instrument product line for Veeco, who sold dozens of the instruments to the manufacturer. The new technology was spun off and acquired by Thermo Scientific, one of the largest scientific instrument providers in the world.
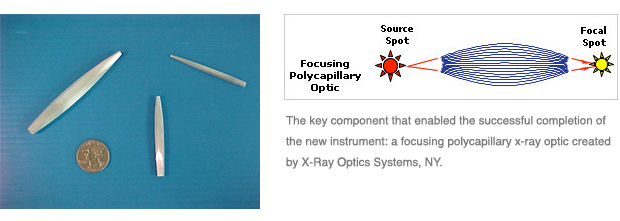
Testimonial
“Don Kloos aided the birth of the analytical technique micro x-ray fluorescence. Made possible by the development of practical x-ray optics, Veeco Instruments NY was trying to move from supplying low-end general purpose XRF instruments at ~$30k, to providing the first of the modern breed of optic-enhanced µXRF instruments. As a consultant Mr. Kloos identified the first commercial niche application, manufacturers of disk drive read/write heads. He assisted both the customers and Veeco to understand the benefits and necessary product specs. This was also critical for XOS, as the supplier of the enabling x-ray optics. The result was a strong product launch and market penetration worldwide for Veeco at a price point of $130k, providing much better margins. This led to the acquisition of the Veeco division by Thermo Scientific, the industry leader in scientific instruments.”
David Gibson, President and CEO, X-Ray Optical Systems, Inc.
